Abstract
Introduction Elranatamab is a bispecific antibody against BCMA and CD3 currently in clinical development for patients with relapsed or refractory multiple myeloma (RRMM). Cytokine release syndrome (CRS) is the most common adverse event associated with elranatamab treatment. PK/PD modeling characterized early clinical data and was used to select priming regimens with target rates, grades, and predictable profile which was then tested and validated in the clinic. This work presents pooled analysis of CRS across 4 clinical studies leading to the selection and validation of the optimal priming regimen for elranatamab as a 2 step-up priming regimen 12 mg on Day 1, 32 mg on Day 4 with first full dose of 76 mg on Day 8.
Methods Elranatamab pharmacologically optimal dose for RRMM patients (76 mg QW) was determined based on data from the first-in-patient study MagnetisMM-1. Dose escalation cohorts in MagnetisMM-1 (SC 80-1000 µg/kg) did not utilize a priming regimen. To mitigate the risk for CRS observed during dose escalation, several priming dose regimens were evaluated across 4 clinical studies (2 Phase 1 studies MagnetisMM-1, MagnetisMM-2; 1 Phase 1/2 Study MagnetisMM-9; 1 Phase 2 Study MagnetisMM-3). Logistic regression analysis correlated maximum elrantamab serum concentration at 24 hours (Cmax-24h) to the probability of All Grades and Grade ≥2 CRS. Elrantamab exposure was correlated to maximum cytokine increase to ensure reasonable cytokine stimulation with the first priming dose. Model simulations predicted CRS rates and profile with potential 2-dose step-up priming regimens. The priming regimens evaluated include a single dose priming regimen of 44 mg / 76 mg (Days 1 and 8) with premedications [44 + pre] or without premedications [44 no pre], a 2-dose step-up priming regimen of 12 mg / 32 mg / 76 mg with premedications [12/32 + pre], and a 2-dose step-up priming regimen of 4 mg / 20 mg / 76 mg with premedications [4/20 + pre]. Step-up doses in the 2-dose priming regimens were administered on Days 1 and 4 with the first full dose on Day 8. Premedications included corticosteroid, antihistamine, and anti-pyretic for all priming doses and the first full dose.
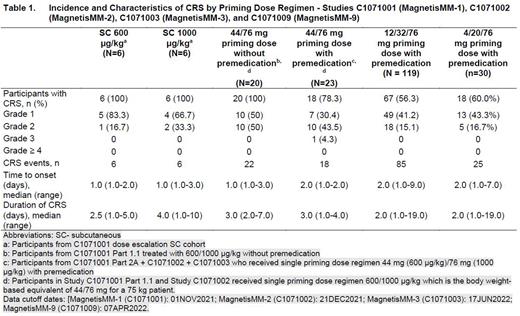
Results During SC dose escalation in MagnetisMM-1 (no priming), All Grade CRS was observed in 73.3% (22/30 pts). CRS was observed in all participants in the 600 and 1000 µg/kg cohorts (n = 6 each). Two participants in 1000 μg/kg cohort experienced prolonged CRS (10 days each). A single priming dose without premedication (44 no pre) resulted in CRS in 100% of pts (n = 20), with 50% G1, 50% G2. Addition of premedication (44 + pre) resulted in All Grade CRS in 78.3% (18//23) (30.4% Grade 1, 43.5% Grade 2 and 4.3% Grade 3). All CRS events with 44 + pre were observed after the first dose. The single priming dose regimens resulted in a median duration of CRS of 3 days for both cohorts with no CRS event with duration > 7 days with 44 + pre associated with a shorter duration range (1 to 4 days) vs 44 no pre (2 to 7 days).
Exposure-CRS modeling described data from dose escalation, 44 no pre, 44 + pre regimens in Studies MagnetisMM-1 and MagnetisMM-2. Model predictions indicated that a 2-dose step-up regimen (12/32 + pre) would be associated with CRS rates of 55% All Grades and 14% Grade ≥ 2. Exposure-cytokine analysis indicated reasonable cytokine elevation at the predicted exposure at 12 mg and lower increases at doses < 12 mg suggesting that first step-up doses < 12 mg might not provide sufficient priming. Consistent with model predictions, CRS incidence in MagnetisMM-3 for 12/32 + pre was 56.3% (67/119), all events were Grade 1 (41.2%, 49/119) or Grade 2 (15.1%, 18/119) with no Grade 3 events. Timing of CRS events with 12/32 + pre was predictable occurring primarily after the first dose (44.5%) or the second dose (20.2%). Only occasional events were observed after the third (5.9%) or later doses (0.8%). To broaden the understanding of elranatamab CRS profile, another 2-dose step up regimen 4/20 + pre was evaluated in MagnetisMM-9. Preliminary data from 4/20 + pre regimen indicates a similar CRS incidence as 12/32 + pre (Table 1), however, more CRS events were observed with the 3rd (13.3%) and later (10%) doses compared to 12/32 + pre.
Conclusions: A 2-dose step-up priming regimen of 12 mg / 32 mg with premedications is the optimized elranatamab priming dosing regimen given a) the observed incidence of All Grades CRS and Grade ≥ 2 CRS and b) the predictable timing and manageable profile of CRS with this regimen.
Disclosures
Elmeliegy:Pfizer: Current Employment. Viqueira:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Vandendries:Pfizer: Current Employment, Current equity holder in publicly-traded company. Hickman:Pfizer: Current Employment. Hibma:Pfizer: Current Employment. Lon:Pfizer: Current Employment. Piscitelli:Pfizer: Current Employment. Soltantabar:Pfizer: Current Employment. Wang:Pfizer: Current Employment. Jiang:Pfizer: Current Employment, Current equity holder in publicly-traded company. Finn:Pfizer: Current Employment, Current equity holder in publicly-traded company. Czibere:Pfizer: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal